| Structure/class |
- Sedative-hypnotic
|
| Pharmacodynamics |
- Binds to the benzodiazepine site of the GABA-A receptor.
- Main function is to increase the efficiency of GABAergic synaptic inhibition.
- Facilitates increased frequency (c.f. barbiturates, that increase duration) of the opening of chloride channel.
- Benzodiazepines are unable to open the channel itself. (c.f. barbiturates, which can open the channels themselves at higher doses)
|
| Absorption/Administration |
- PO, IV, IM, PR
|
| Distribution |
- Lipid soluble drug
- Very strongly bound to plasma proteins.
|
| Metabolism |
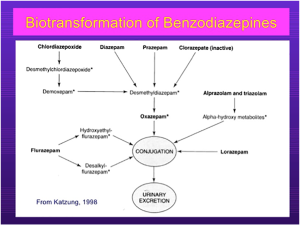
- Refer to the diagram above for benzodiazepine metabolism. The asterisks indicate active metabolites. Note that diazepam is metabolized to desmethymdiazepam, which is active.
- Lorazepam has no active metabolite.
- Note that hepatic metabolism accounts for clearance of all benzodiazepines.
- Most BZDs will undergo phase I reaction, catalyzed by CYP450 systems. The metabolites are then conjugated to form glucuronides and excreted.
- However, many phase I metabolites are pharmacologically active, e.g. desmethyldiazepam. This means that the T ½ of diazepam is 40 hours.
- Triazolam is metabolized to its α-hydroxy metabolites that do have activity, but they are rapidly conjugated.
- Note that the metabolism of diazepam, midazolam and alprazolam may be induced/inhibited by other drugs.
- In patients who are elderly and have severe disease, these drugs may have longer elimination half-lives.
In summary, BZDs undergo phase I and phase II reactions in the liver and are excreted by the kidneys. Liver disease affects T ½ but renal disease does not. |
| Excretion |
- Renal
|
| Indications |
- Can be used in ED to treat status epilepticus
- Procedural sedation
- Aids induction prior to intubation
- Alcohol withdrawal (Delirium tremens)
- Other – muscle relaxation, sedative hypnotic in agitated patient, post intubation sedation.
|
| Contraindications |
|
| Special precautions |
- Elderly and those with pre-existing hepatic disease.
|
| Interactions |
- Inhibitors of CYP 450 (e.g. amiodarone, cimetidine and antibiotics) may increase duration.
- Inducers will reduce duration of action.
- Additive toxicity (CVS and respiratory depression) with opioids.
- Also additive toxicity with alcohol and other typical anti-psychotics (Esp. the phenothiazines)
- It is antagonized by flumazenil
|
| Adverse events |
- Extension of its pharmacologic properties
- Oversedation, leading to respiratory depression (especially in patients with pre-existing pulmonary disease). It is a dose related inhibition of the respiratory centre.
- CVS Depression (especially in patients who are hypovolemic/CCF/pre-existing CVS disease) due to inhibition of vasomotor centers.
- CVS collapse is possible.
- Effects more marked with IV use.
- Tolerance (more drug needed to produce same effect)
- Cross tolerance occurs with EtoH
- May be due to down-regulation of BZD receptor
- Dependence, both physiological and psychological
- Characterized by anxiety, insomnia, CNS excitability and seizures in severe cases.
- Higher dose/drugs with short T ½ and abrupt cessation leads to withdrawal symptoms.
|
| Dosing/administration |
- For status: 0.15mg/kg IV; or 10mg PR and repeat in 10 minutes.
|
| Toxicology |
- Flumazenil is the direct antidote
- It is a competitive antagonist of benzodiazepines at the BZD receptor. It also reduces GABA binding. This leads to reduced chloride entry into the cell, and therefore reduced hyperpolarization and increased excitability.
- Indications for flumazenil: a) avoid intubation after BZD overdose; b) reversal of BZD after procedural sedation; and c) diagnostic tool.
- Adverse effects as follows:
- Very short T ½ (duration of action is 1-3 hours) and therefore may need repeated dosing.
- Unpredictable effect on antagonism of respiratory depression (that is, giving flumazenil may not reverse respiratory effects of BZDs)
- Risk of precipitating seizures in mixed OD
- Risk of precipitating withdrawal symptoms
- Risk of precipitating seizures in a patient taking BZDs for epilepsy.
- Due to unpredictable effect on antagonism, ongoing CVS/respiratory monitoring is required when used.
|
| Withdrawal syndrome |
|
| Special notes |
|